Introduction
The matter of babies produced from three parents has been floating about the media as of late and really grabbed my attention, as it seems like quite a futuristic procedure that I think will only gain momentum. The moniker “three parent babies” refers to babies who have the DNA of their mother and father, plus a little from a donor. This extra bit comes in handy when the mother’s mitochondria has some sort of mutation or defect that can be passed on to offspring. A healthy female donor’s mitochondria is incorporated instead, allowing for the creation of a baby with healthy mitochondria. The mitochondria are the little machines governing cellular energy metabolism and respiration, so defects of the mitochondria can be catastrophically calamitous.
The concept of three parent babies is not new. In fact, it was in the late 1990’s that embryologist Jacques Cohen first experimented with cytoplasmic transfer to overcome mutations in the mother’s mitochondria that would produce a defective zygote. However, this procedure was deemed risky since it involved adding cytoplasm (including the organelles) from a donor egg to the recipient, which boosted the number of healthy mitochondria in the egg but the defective mitochondria remained- making it likely to still pass on defects. Indeed, of about 30 babies born in the nineties using cytoplasmic transfer technology, there were babies born with sex-linked autism disorders, Turner syndrome, and developmental disorders. This caused the U.S Food and Drug Administration (FDA) to ban the procedure until it was proved safe. It was in 2016 that the Human Fertilization and Embryology Authorization (HFEA) approved the use of mitochondrial transfer therapy in the UK, when it was shown that only about 2% of the defective mitochondrial DNA remained in the egg after receiving mitochondria from the donor. This was low enough that the healthy mitochondria vastly outnumbered the defective ones, making transmission of mitochondrial illness unlikely. The newer technology involved as much removal of the mother’s mitochondria as possible, leaving minimal defective mitochondria in the egg.
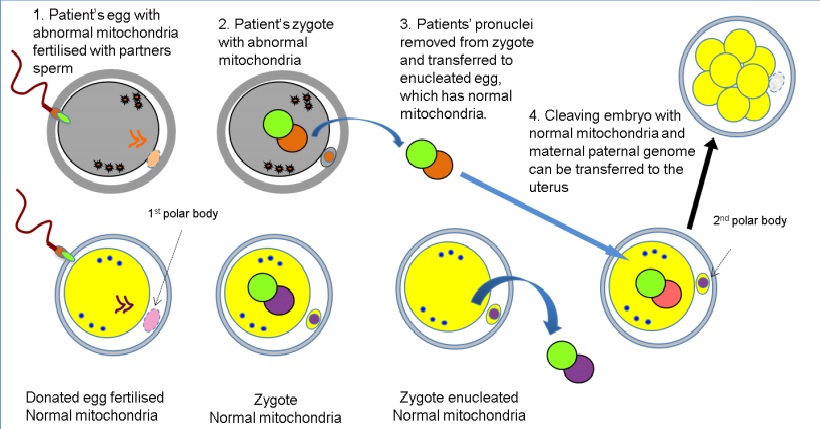
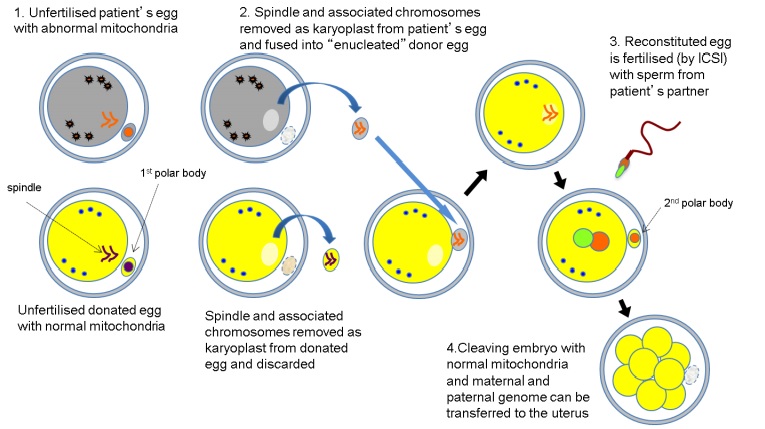
There are two principal techniques to the creation of three parent babies: pronuclear transfer and spindle transfer. The objective of both techniques is to produce a zygote with healthy mitochondria from a donor, with the defective or diseased mitochondria of the mother evicted. In this way, a zygote can be created that will (hopefully) develop into a healthy baby without the burden of the mother’s faulty mitochondria.
Pronuclear transfer is done as follows: The mother’s egg and a donor’s egg are fertilized by the father’s sperm. The pronuclei (sperm and egg nuclei not yet fused together) of the donor are removed from the donor cell, and the pronuclei of the mum and dad are transferred into the donor egg cell. Then the zygote is implanted into the mum’s uterus and can grow into a baby.
Pronuclear transfer technique used in mitochondrial transfer technique. Human Fertilization and Embryology Authority Report, Andy Greenfield. 2014.
Spindle transfer is done as follows: The mitotic spindle and associated chromosomes of the mother’s egg are transferred into the egg of a donor, which has had its spindle and chromosomes removed. The egg is then fertilized with the father’s sperm, transferred to the mother’s uterus and allowed to develop.
Spindle transfer technique used in mitochondrial transfer therapy. Human Fertilization and Embryology Authority Report, Andy Greenfield. 2014.
The Lowdown on Mitochondrial DNA
The DNA contained in mitochondria codes for and controls 37 genes, and is inherited down in matrilineal succession– mother to daughter to granddaughter and so on. Boys receive mitochondrial DNA from their mum, but do not pass it on further (sorry boys). This is used by some as the basis for the argument that only male babies should be brought to term using mitochondrial replacement therapy, as there is no risk of donor mitochondrial DNA being passed on from a male, especially if he ends up developing some disorder stemming from mitochondrial malfunction.
Physical features such as eye colour, hair colour, and height are not determined from mitochondrial DNA, so the genetic input from the third party donor is minimal. This means that the genes of the third party donor would not affect the baby’s physical outward appearance, just the way they use energy in their cells.
The Question of Ethics
So now to get to the crux of the matter: is the creation of 3 parent babies ethical? Me, I am on the fence about this because there are valid pros and cons that must both be considered, and while I feel that mitochondrial transfer therapy can be a godsend for some folks, it carries risks that can’t be overlooked.
- Defective mitochondria can still make it into the zygote. While the transfer of pronuclei to the healthy donor egg is done as carefully as possible, a small amount of defective mitochondria can still make it into the donor egg. While such a small amount of defective mitochondria as about 2% of the total is unlikely to cause issues in the child, this can be problematic if the resultant child is a female. This is so because the residual mutant mitochondria can affect future generations, even if the female in question is unaffected, as the mitochondria is passed down and replicated. For example, heteroplasmy in the mitochondria may cause a variety of diseases, usually expressed in adulthood. Due to this risk, many guidelines exist allowing only male embryos to be brought to term to rule out the potential passing-on of defective mitochondrial DNA.
- Location location location. A baby boy was born in April 2016 who had been developed from spindle transfer therapy. His mum carries Leigh Syndrome, which affects the central nervous system and causes a host of problems from delayed development to seizures to impaired kidney function. The spindle transfer was performed in Mexico, where healthcare standards and regulations are notoriously unpoliced. In fact, the American lead specialist, John Zhang, has received flack for going to Mexico to work on this project, as this was seen as attempting to sidestep American restrictions on mitochondrial transfer therapy. Mitochondrial transfer therapy is allowed in the UK (conditions apply) as of 2016, although the FDA is currently blocking such experimental. Therapy procedures in the USA due to uncertainty of outcome and questionable health of babies born from mitochondrial transfer. It remains an issue of proving the therapy safe, and following up with babies of mitochondrial transfer, before the USA and other countries might approve this procedure.
- Slippery slope. An argument against mitochondrial transfer therapy is based on the question “how far is too far?” That is, if we are okay with modifying the germline to prevent certain diseases, why not others? In fact, why not increase positive traits like athletic ability, intelligence, or beauty? Even though mitochondrial DNA is involved in mechanisms of cellular energy usage and not in features such as hair lusciousness, the worry that mitochondrial transfer therapy is a step in the direction of creating “designer babies” is another roadblock to getting the therapy approved.
Business baby that may have been designed to be more intelligent than you or me. Credit: Paul Inkles (CC by 2.0)
Conclusion
Mitochondrial transfer therapy holds the potential to give healthy children to women who have mutant mitochondria, but is riddled with ethical concerns. Health safety, restrictions, ethics, and even other concerns like questions of identity dog the progress of establishing the legality of this therapy. We shall have to wait and see what transpires in the future, as further testing of mitochondrial transfer therapy gets underway.


Recent Comments